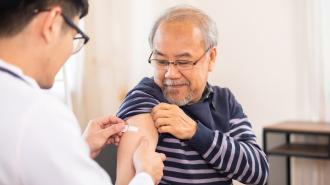
After decades of disappointment, the world finally has its first vaccine for respiratory syncytial virus (RSV), a potentially deadly infection that hospitalizes 120,000 seniors in the US every year.
The challenge: RSV is a very common virus, and for most adults, an infection feels similar to a bad cold: you might cough and sneeze a lot, have a headache, and just want to spend a few days in bed. After that, you’re back to your old self.
However, RSV can cause serious lower respiratory tract diseases (LRTDs), such as pneumonia and bronchiolitis. In some cases, it can even be fatal, and infants and seniors are at the highest risk of severe infections.
RSV infections hospitalize 120,000 seniors in the US every year.
An RSV vaccine: The hunt for a vaccine for RSV began in the 1960s, but the first major clinical trial ended in tragedy. Rather than reducing the severity of the disease, the antibodies created by that vaccine made it worse; two children who got the vaccine died when they contracted RSV.
Subsequently, scientists discovered the problem was the ability of the virus’ F protein to change shape after fusing with cells.
Now, after decades of research, the wait for a safe and effective vaccine for RSV is finally coming to an end, as the FDA has approved a shot developed by UK drugmaker GSK, for use in people over the age of 60.
“Today marks a turning point in our effort to reduce the significant burden of RSV,” said Tony Wood, GSK’s chief scientific officer.
How it works: The vaccine, called Arexvy, is a “recombinant subunit” vaccine, meaning it uses a harmless bit of protein from the virus to trigger an immune response. Specifically, GSK’s shot contains the version of the F protein before it fuses with cells, stabilized in that form so that the vaccine produces only the right kind of antibodies.
The FDA’s approval was largely based on a phase 3 trial involving about 25,000 adults over the age of 60. During it, GSK’s RSV vaccine was found to reduce the risk of RSV-caused LRTD by 82.6% compared to a placebo.
“Today marks a turning point in our effort to reduce the significant burden of RSV.”
Tony Wood
The vaccine also reduced the risk of severe disease — defined as two or more symptoms of LRTD, an assessment of a “severe” case by a trial investigator, or the need for supportive therapy, such as ventilation — by 94.1%.
The most common adverse effects were injection site pain, fatigue, muscle pain, and headache, and they were short-lived and generally mild to moderate.
Now that the FDA has approved Arexvy, the CDC will debate the details, such as how it should be administered and when it should become available. That decision will likely come in June, and GSK anticipates delivering the shot to pharmacies and clinics in Fall 2023.
The big picture: GSK’s vaccine for RSV is the first, but it probably won’t be the only one for long: both Pfizer and Moderna have both announced promising data from phase 3 trials of their own jabs in older adults, and both companies expect to find out in 2023 whether they’ve been approved by the FDA.
“There’s just the broad excitement of finally, after all these years, having good options emerging for RSV.”
Phil Dormitzer
Meanwhile, Pfizer’s shot was also shown to reduce the risk of severe RSV infections in infants by 81.8% when administered to their mothers during pregnancy. The FDA is also reviewing a promising new antibody treatment, developed by French pharma company Sanofi, that’s given to infants to prevent RSV infections.
If either of those medicines are approved, we could soon have a way to protect both age groups at highest risk of life-threatening RSV.
“There’s just the broad excitement of finally, after all these years, having good options emerging for RSV,” Phil Dormitzer, GSK’s senior VP and global head of vaccines R&D, told STAT.
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].