Researchers at Israel’s Weizmann Institute of Science have effectively treated mice for heart attack before the heart attack ever happened.
The mice had a gene in their cardiomyocytes — the heart cells responsible for the crucial contractions of life — toggled on and then off again, which allowed them to recover from heart damage that occurred months after their treatment.
While barely on the horizon for human use, the study, published in Nature Cardiovascular Research, provides a proof-of-concept for the field of regenerative heart therapies, which seek to restore the function lost in a heart attack or other cardiovascular diseases.
“The data made our jaws drop,” Eldad Tzahor, whose lab at Weizmann studies regenerative medicine, said. “We had found a cardiac fountain of youth in those mice, a novel way of making the heart younger and stronger.”
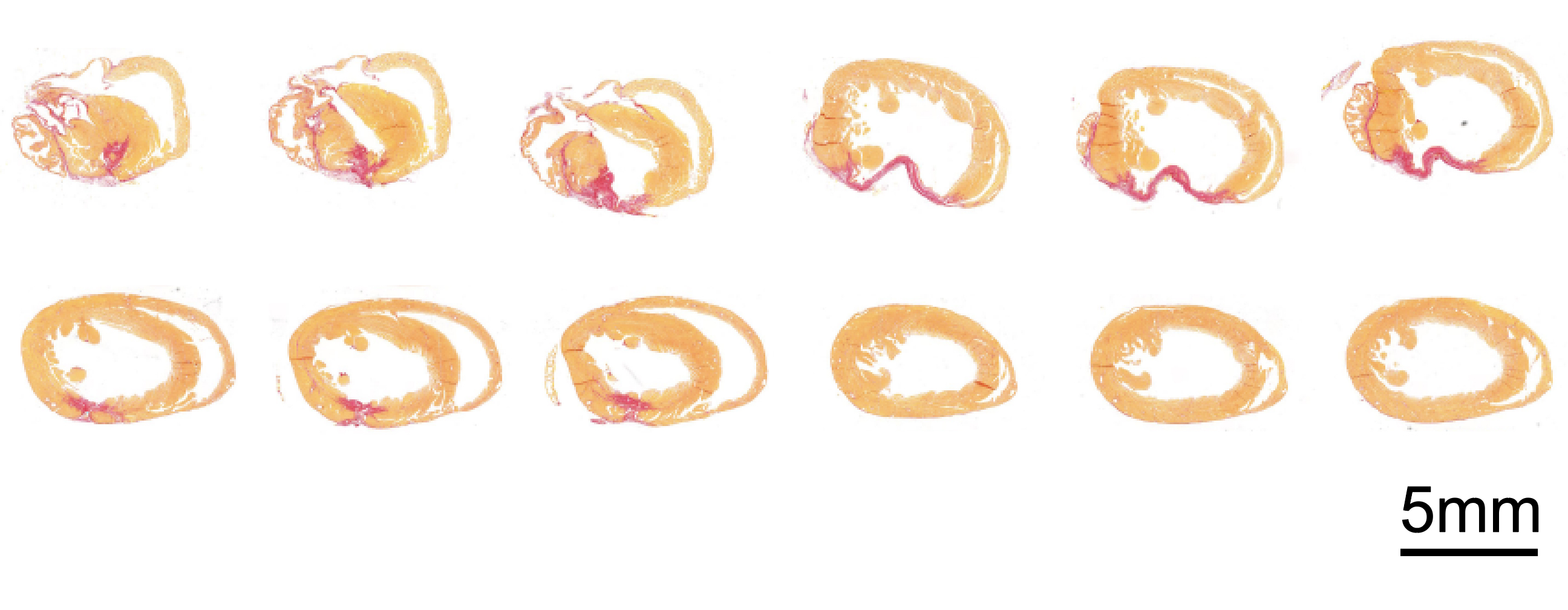
Heart beats: Cardiovascular disease is the leading cause of human death: the WHO estimates that it accounted for 17.9 million deaths in 2019 alone — almost a third of all deaths.
Of that grim number, the lion’s share are due to strokes and heart attacks.
A heart attack occurs when the flow of blood, richly red with oxygen, is severely restricted, usually due to buildup in the arteries of substances like cholesterol and fat.
Cut off, the cardiac muscle begins to die; if a person survives the attack, they’ll be left with permanent damage, likely reducing heart function for life. Cardiomyocytes and other heart tissues do not regenerate naturally.
But labs around the world are exploring new ways to potentially regenerate and repair damaged hearts.
At the University of Texas Southwestern, researchers have used a more targeted form of CRISPR, called base editing, to alter enzyme expression, which led to mice recovering function after a heart attack. And a team at King’s College London is using microRNA to try to stimulate the growth of new heart cells in pigs.
The ability to regenerate or repair cardiomyocytes would be one of medical science’s greatest triumphs if it could be achieved. But a preemptive treatment may be even better.
Controlling cardiomyocytes: The therapy developed at Weizmann hinges on a gene called ERBB2. In previous studies, the researchers had already found that triggering ERBB2 (a good name for the next adorable Star Wars droid) caused cardiomyocytes to divide and replicate — an ability the cells usually lose at birth.
“During fetal development, our cells are assigned their different roles – nerves, cornea, heart muscle etc. – through a process called differentiation,” said study lead Avraham Shakked.
You can think of differentiation as a spectrum. On one extreme are stem cells, which can become any kind of cell. On the other end are cells like cardiomyocytes, which are locked in to very specific jobs; they can no longer divide and multiply once they’ve been assigned their task.
“They’re very effective in their function, but the tissue they comprise does not regenerate naturally,” Shakked said.
But there’s a reason that the heart ends up like this: it works better that way. When ERBB2 was switched on in previous studies, it did trigger cell division — but at the cost of heart function.
That was because the freshly-christened cardiomyocytes “de-differentiated,” coming into being more similar to a fetal heart than an adult’s, and so were not as adept on the job. When ERBB2 was switched back off, the cardiomyocytes became more specialized again, and heart function improved.
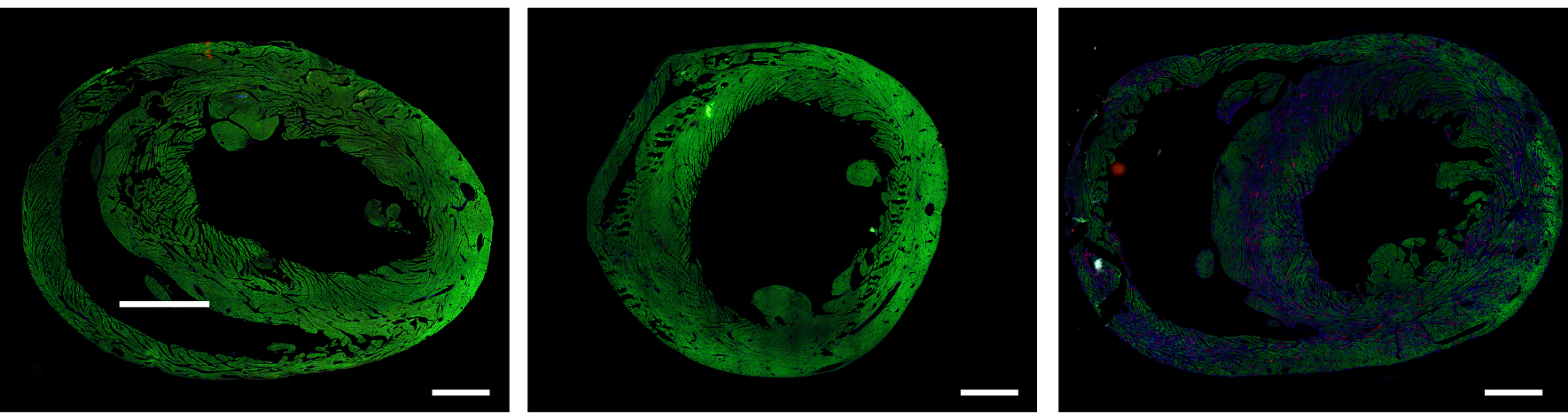
Strangely, researchers found that the modified hearts — while once again identical in performance — kept a significantly different expression of genes than the control hearts.
“It was surprising and curious,” Shakked said. They had assumed the cardiomyocytes would have returned to normal, but instead they had different genetic patterns, overexpressing or under-expressing different genes than the control hearts.
“In other words, we found long-term effects.”
Treating heart attacks before they happen: Those long-term effects sparked an idea.
“It made us think that ERBB2 wasn’t just a switch that prevents differentiation, but part of a mechanism that could make the heart younger and more resilient,” Tzahor said.
To find out, the team switched on ERBB2 in a group of healthy mice, letting the gene cook for a few weeks, before turning it back off. They then caused cardiac damage — essentially, a heart attack — in the ERBB2 mice and control mice. The mice who had undergone the on-off ERBB2 treatment recovered, while the others did not.
One mouse, which had ERBB2 toggled at three months old, recovered from major injury two months later.
“If we translate this to human years, it’s comparable to an 18-year-old getting treatment that allows that person to survive a heart attack at age 50,” Tzahor said.
“The data made our jaws drop. We had found a cardiac fountain of youth in those mice, a novel way of making the heart younger and stronger.”
Eldad Tzahor
What it means for people: Exciting as the idea of preemptive heart attack treatment may be, there are major blank spots to be filled and potentially dangerous complications to be conquered.
The therapy, as is, would be simply unacceptable for human beings, since it first lowers the functionality of healthy cardiomyocytes to hedge against theoretical future damage — a tough trade to sell, clinically speaking.
“From a clinical perspective, this is an extreme and drastic intervention,” Tzahor said.
The team’s next steps are figuring out just how, exactly, the treatment could work and the mechanisms behind it.
“Still, at least in principle, our research might lead to a way of treating people who have a high risk of heart attack, before these attacks even happen.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].