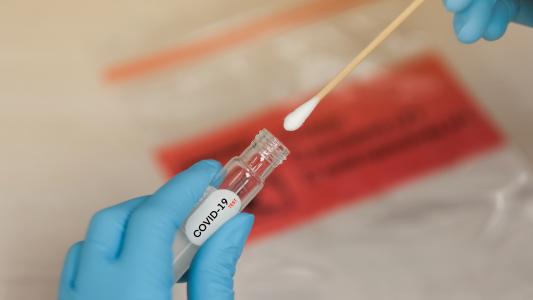
Healthcare providers now have a new coronavirus test at their disposal.
On March 27, the Food and Drug Administration (FDA) issued emergency approval for Abbott Laboratories’ rapid COVID-19 test, which delivers results in as few as five minutes — a dramatic improvement over current testing times.
The Need for a New Coronavirus Test
The average wait time for coronavirus test results in the United States is four or five days. Some people, however, say they’ve had to wait days longer for their results.
This has left many potential coronavirus patients in lockdown limbo.
“Having results quickly provides important peace of mind.”
Robert S. Levy
Those who got tested because they have coronavirus symptoms — and not just because they might have been exposed to the virus — are stuck wondering whether they should be trying to power through a bad flu at home or visiting a hospital because they have the coronavirus.
Symptomless people who still have jobs to go to, meanwhile, are left unsure whether they might spread the virus to their coworkers if they go to work. Those stuck at home, meanwhile, are left wondering if they should be limiting their interactions with family members or roommates.
Thanks to Abbott’s new coronavirus test, though, the era of long wait times for results could be coming to an end.
Abbott’s COVID-19 Solution
Abbott’s new coronavirus test runs on the company’s ID NOW platform. An estimated 18,000 of these toaster-sized devices are already in use throughout the U.S., with doctors and other healthcare professionals using them to diagnose the flu and other illnesses.
To prep the device for coronavirus detection, a healthcare worker first inserts a $40 test cartridge. They then feed the system a patient’s specimen sample, which is taken from the back of their throat and nose.
The chemicals in the cartridge break down the sample’s cells, releasing their genetic material. If the chemicals encounter the coronavirus’s genetic material, they replicate it, and a few molecules quickly become a billion.
That increase makes the virus detectable, and within five minutes of starting the process, the system can deliver positive results. Negative results take a little longer, up to 13 minutes.
Widespread Availability, Fast Answers
Abbott said in a recent press release that it’s working to manufacture and deliver 50,000 tests per day initially, and eventually, it hopes to produce more than a million of the coronavirus tests every month.
On March 31, three American Family Care (AFC) urgent care facilities in New York became the first to offer patients Abbott’s new coronavirus test — giving them much-needed direction on how to act during the coronavirus crisis.
“Having results quickly provides important peace of mind for those who test negative… those who test positive then know to immediately self-quarantine or seek further medical care,” AFC’s Dr. Robert S. Levy said in a statement.