A first-of-its-kind AI-powered brain implant has restored movement and feeling in a man with quadriplegia — and some of the regained abilities remain even when the device isn’t on.
“This is the first time the brain, body, and spinal cord have been linked together electronically in a paralyzed human to restore lasting movement and sensation,” said Chad Bouton, trial leader and vice president of advanced engineering at Northwell Health.
The challenge: Nearly 5.4 million people in the US are living with some level of paralysis. Keith Thomas, a 45 year old from New York, is one of them — he lost the ability to feel or move from his chest down in a diving accident in 2020.
“I dove in aggressively, as usual, and then I just blacked out,” said Thomas. “Next thing you know, there was a helicopter on the front lawn. The next day, I couldn’t even move.”
“There was a time that I didn’t know if I was even going to live, or if I wanted to, frankly.”
Keith Thomas
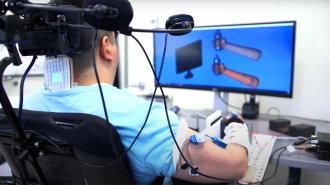
An AI-powered brain implant: In 2021, Thomas agreed to participate in a clinical trial in which doctors would use a combination of implants, electrodes, and AI algorithms to create a bridge between his brain and limbs.
Thanks to that tech, he can now move his arms and even feel sensations again.
“There was a time that I didn’t know if I was even going to live, or if I wanted to, frankly,” said Thomas. “And now, I can feel the touch of someone holding my hand. It’s overwhelming.”
15-hour surgery, awake: At the start of the trial, Bouton’s team used functional MRI (fMRI) to create a map of Thomas’ brain, identifying areas linked to arm movement and hand sensation.
In March 2023, Thomas underwent a 15-hour surgery to have implants placed in his brain. To ensure they went in exactly the right places, he was kept awake during the operation and let the doctors know what he felt in his arms and hands as they probed different parts of his brain.
“We inserted two chips in the area responsible for movement and three more in the part of the brain responsible for touch and feeling in the fingers,” said lead surgeon Ashesh Mehta.
After the operation, researchers used two ports they’d placed in Thomas’ skull to connect his brain implants to a computer. The computer was then connected to electrode patches placed over his spine and forearm muscles.
AI algorithms were trained to detect and interpret the brain signals produced when Thomas thinks about moving his arms. The computer then sends signals to the electrodes over his arm — these stimulate his muscles in a way that gets his body to actually perform the movements he’s thinking about.
This type of system is called a neural bypass, or “neural bridge,” because it connects the brain and limbs while bypassing the site of a spinal injury. It’s been used to restore movement in people with paralysis before, but Thomas’ team didn’t stop with just one neural bypass.
To restore some sensation in Thomas’ hands, the researchers connected the computer to tiny sensors on his fingertips and palm. AI algorithms translate the sensor data into signals that stimulate his brain in a way that lets him feel when his hand is being touched.
“That electrical stimulation, we believe, is awakening circuits that have been damaged and dormant for three years.”
Chad Bouton
Thought-driven therapy: The double neural bypass isn’t just an assistive device — it’s also designed to help rehabilitate Thomas.
While he uses the AI-powered brain implant, the electrode patch over his spine also stimulates his spinal cord. This appears to be helping reestablish the natural connection between his brain, spine, and limbs — he can now feel some sensations in his arms and hands even when the system is off, and his arm strength has doubled since the start of the trial.
“That electrical stimulation, we believe, is awakening circuits that have been damaged and dormant for three years,” Bouton told TIME.
“This type of thought-driven therapy is a game-changer.”
Chad Bouton
Looking ahead: The plan is to enroll two more participants in the clinical trial. Even if others respond as well as Thomas has and the AI-powered brain implant is approved by the FDA, the high cost and invasiveness of the procedure may limit its adoption, at least initially.
To overcome that, Bouton is also developing a version that relies solely on non-invasive electrodes — no brain surgery required. He thinks it could potentially be used by people with less severe paralysis, such as stroke survivors.
“This type of thought-driven therapy is a game-changer,” said Bouton. “Our goal is to use this technology one day to give people living with paralysis the ability to live fuller, more independent lives.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].