Our lives, in the modern age, are dominated by the technologies of electronics and electrical energy. Our worldwide need for large amounts of continuous power underscores the need for increased efficiency across the board: from energy generation to transmission to consumption. In every step of that process, energy loss is a problem, as the very act of pushing electrons through a current-carrying wire is an energy-losing proposition, owing to the electrical phenomenon of resistance. There’s only one physical circumstance where current can be transmitted with no resistance: when your material is superconducting. Superconductors today have a wide variety of applications, from MRI machines to particle accelerators to magnetic fusion devices and many, many others.
At present, however, the only known materials that superconduct do so under extreme conditions: very low temperatures. The “holy grail” of superconducting research is to find a material that would superconduct under normal conditions: at room temperature and ambient pressures. If we could discover one and implement it on a wide scale, we could eliminate all the problems of energy loss and stray heat: problems that every consumer and device manufacturer must presently reckon with. In late July of 2023, a claim that a new material — known as LK-99 — is, in fact, that long-sought-after room temperature superconductor. But is it real? Lots of you have written to me about it, including Rob Chapman-Smith and Clint Sears, who’ve asked:
“Where are we right now in terms of how it’s looking because it’s been a roller coaster in real-time of hope and failure… [S]cientifically speaking, how would you go and replicate this, how would we know the replication is correct, how would we know it’s incorrect?”
Whenever any claim is made that, if true, would change the world, it’s vital to understand not only what we know at present, but what we’ll need to know to determine precisely what is and isn’t true. Let’s dive into the science and find out!

What is a superconductor?
Every material, when you try to pass an electrical current through it (i.e., when you try to cause electrons to move within it), exhibits some form of resistance. That’s because every material, naturally, has a property known as resistivity to it: where the resistivity of your material multiplied by its length and divided by its cross-sectional area equals what we conventionally call resistance. (For those of you who learned Ohm’s law, V = IR, V is the voltage, I is the current, and R is the resistance.) If you build a shorter, thicker wire, the resistance goes down; if you build a longer, thinner wire, the resistance goes up.
But resistivity, under most circumstances, isn’t an absolute property of any such material, but rather depends on the temperature of that material. At higher temperatures, the molecules, atoms, and even the subatomic particles within the atoms move around more rapidly, and the resistivity gets larger the higher your temperature gets. However, the opposite is also true: at lower temperatures, the internal particles within it move more slowly, have less energy-per-particle, and interact less in general, and the resistivity drops.
For most materials, that’s the end of the story: you’d need to reach absolute zero — a physically unattainable state — to get zero resistivity, and hence, zero resistance irrespective of the other properties of your material. But for some materials, there’s a critical threshold that you can cool them down to or below, and when you reach that threshold, the resistivity and resistance all at once plummet to zero. Those materials are superconductors, and that state of zero resistivity and resistance is a superconducting state.

From a physical point of view, what makes a superconductor special?
Rather than go down the rabbit hole of what you can accomplish and create when you have a superconductor — as most of those possibilities are yet undiscovered — I’d rather help you understand what allows a material to be superconducting from a physics perspective. Under normal circumstances, even within a conductor, simply having electric charges move through it prevents a material from reaching a superconducting state.
Think about why this must be the case. If you have an electric charge in motion, even just one of them, it’s going to create a magnetic field around it: that’s one of the fundamental rules of electromagnetism. Electric currents create magnetic fields, and if the magnetic field inside your conductor changes at all, that changing magnetic field will affect the motion of any moving charges within it: a phenomenon that always resists the motion of the electric charge.
In other words, there’s a requirement for “perfect conductivity” that isn’t generally appreciated: the magnetic field inside your conductor cannot change. If all we had were classical (i.e., Maxwell’s) electromagnetism, perfect conductivity would be a physical impossibility, because a current is simply, by definition, generated by moving electric charges. However, there’s an inherently quantum effect — the Meissner effect — that can arise for certain materials: where all magnetic fields inside a conductor are expelled. This makes the magnetic field inside your conductor zero for any current that flows through it. If you expel your magnetic fields, your conductor can begin behaving as a superconductor, with zero electrical resistance.

How long have we known about superconductivity?
Believe it or not, superconductivity was discovered experimentally long before we had the quantum theory in place that could describe and explain it. Its discovery dates back all the way to 1911, when liquid helium first came into widespread use as a refrigerant. Scientist Heike Onnes was using liquid helium to cool down the element mercury into its solid phase, and was then studying the properties of its electrical resistance. Just as expected, for all conductors, the resistance gradually dropped as the temperature dropped, but only up until a point. Abruptly, at a temperature of 4.2 K, the resistance of solid mercury completely disappeared.
Moreover, upon close examination, Onnes found that there was no magnetic field present inside the solid mercury once you crossed below that temperature threshold. Later on, several other materials were shown to exhibit this superconductivity phenomenon, all becoming superconductors at their own unique temperatures:
- lead at 7 K,
- niobium at 10 K,
- niobium nitride at 16 K,
and many other elements and compounds subsequently. Theoretical advances accompanied them, helping physicists understand the quantum mechanisms that cause materials to become superconducting. However, it turns out that not all superconductors behave in exactly the same way.

What makes a material superconduct at higher temperatures?
It turns out that there are two fundamental types of superconductor, creatively named type I and type II superconductors. In a type I superconductor, the transition to the superconducting state happens immediately and all at once: 100% of the internal magnetic field gets expelled and 100% of the material drops to zero electrical resistance. But in a type II superconductor, the material is non-uniform, and magnetic field vortices form within the material when an external magnetic field is applied, particularly at higher field strengths. The magnetic fields are expelled in the region external to each individual vortex, but the magnetic field lines become “pinned” within the material within each magnetic vortex.
Whereas type I superconductivity is normally exhibited only by pure metals (with the only known exceptions being tantalum silicide and boron-doped silicon carbide), type II superconductivity can occur for a wide variety of alloys. Given the sheer number of elements in the periodic table, the unfathomably large number of ways to combine those elements and to bond them together, and the enormous possibilities that exist for doping the material — i.e., selectively replacing some of the elements with others — enables some type II superconductors to do so at temperatures that are far in excess of where a type I superconductor typically reverts to being a normal conductor. Although type II superconductors were experimentally discovered way back in 1935, it wasn’t until the 1980s that the first (relatively) high-temperature superconductors were revealed.
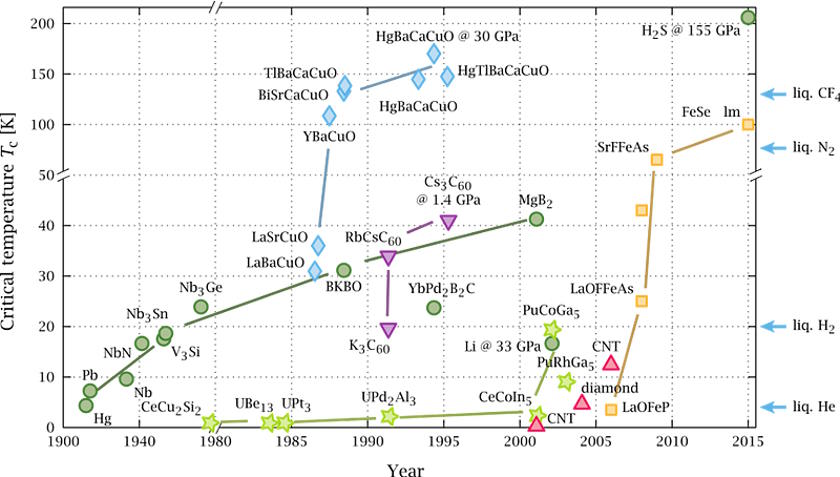
How high of a temperature have the most extreme superconductors reached, while still maintaining their superconducting state?
It started with a simple class of materials: copper oxides. In the mid-1980s, experiments with copper oxides with the elements lanthanum and barium broke the longstanding temperature record by several degrees, being found to superconduct at temperatures greater than 30 K. That record was quickly broken by using strontium instead of barium, and then was broken once again — by a significant margin — by a new material: Yttrium-Barium-Copper-Oxide.
This wasn’t just a standard advance, but rather a huge leap: instead of superconducting at temperatures below ~40 K, which meant that either liquid hydrogen or liquid helium was required, Yttrium-Barium-Copper-Oxide became the first material discovered to superconduct at temperatures above 77 K (it superconducts at 92 K), meaning that you could use the much cheaper liquid nitrogen to cool your device down to superconducting temperatures.
This discovery led to an explosion of superconductivity research, where a variety of materials were introduced and explored, and not only extreme temperatures but also extreme pressures were applied to these systems. However, after a peak in the mid-1990s, no higher-temperature superconductors were found until 2015, when a team in Germany announced that, surprisingly, plain old hydrogen sulfide (H2S, which is like a water molecule but with sulfur substituted for oxygen), when placed under the tremendous pressure of more than 1,500,000 normal Earth atmospheres, superconducted at a whopping temperature of 203 K.
What are our prospects for achieving the “holy grail” of a room-temperature, ambient-pressure superconductor?
Since that 2015 breakthrough, there have been highs and lows in superconductivity research. A class of materials known as lanthanum hydrides and superhydrides have been shown to superconduct under very high pressures at 250-260 K, meaning you could throw them in a conventional freezer or take them to Alaska in the winter (at high pressures) and observe them to superconduct. What’s remarkable about these materials is that theoretical work is what motivates the interest in them, and these new superconductivity temperature records came about as a result of the experimental investigation of these theoretical predictions.
By looking at how the atoms composing the material pack together into a lattice, and then calculating how the electron band structure within the material behaves, potentially interesting behavior that could mean, “yes, this behaves as a type II superconductor at high temperatures” was suggested.
But it isn’t all smooth sailing. While the electron band structure is something that can be calculated and predicted, whether it superconducts or not, and under what exact temperature conditions, is something that can only be measured and confirmed experimentally. Additionally, many adjacent claims of superconductivity, notably conducted by the University of Rochester’s Ranga Dias, have been retracted and/or debunked, as mounting evidence of fraud, plagiarism, irreproducibility, and deception has surfaced over the past couple of years.
So what is LK-99, why is it interesting, does it superconduct at all, and can it superconduct at room temperatures and ambient pressures?
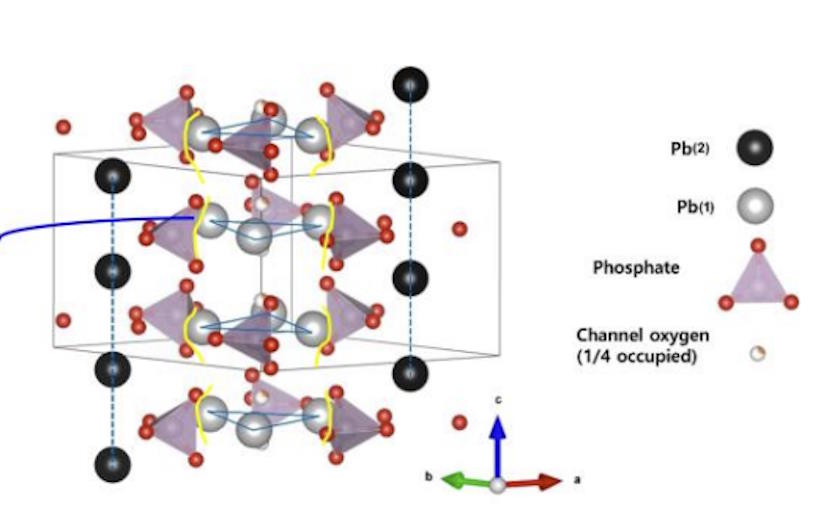
Back in the 1990s, Korea University professor Tong-Shik Choi was researching superconductivity from a theoretical perspective, coming up with a new approach to calculate the electron band levels in materials that suggested a novel set of materials might be good candidates for high-temperature superconductors. Two of Choi’s students at the time were Sukbae Lee and Ji-Hoon Kim, who would eventually extend Choi’s work to suggest that by taking a compound material known as lead-apatite (a mix of lead, phosphorous, and oxygen atoms) and doping it with copper (replacing a fraction of the lead ions within the apatite structure with copper), a material with an interesting electron band structure would result.
Lee and Kim went on to go into industry, with Lee founding a company known as Qcenter and Kim working for a battery materials company before joining Lee at Qcenter. In 2017, Choi died, expressing his desire for his students to find the high-temperature superconductor his theory predicted might exist. The copper-doped lead-apatite is what they’re now calling LK-99. They published two papers, one a three-author paper and one a six-author paper, claiming that it superconducts and exhibits the famed phenomenon of quantum levitation.
And, as is always the case whenever anyone puts forth anything that — if true — would truly change the world, it’s been met with great fanfare, reflecting our hopes that we want this technology to be real and the science to be robust, but also our great fears that it may just be another entry in a long line of preliminary, potentially sloppy results that won’t hold up to scrupulous scrutiny and reproduction attempts.

Because the material they use, copper-doped lead-apatite, is relatively straightforward to create, coupled with the novel theoretical approach underlying the material, many teams have raced to replicate these findings. The open-source nature of the arxiv, the preprint server where the papers have been uploaded, has meant that everything is publicly available immediately, without the need to wait for peer review. The wikipedia page for LK-99 has been tracking (and will continue to track) replication efforts, and a series of very interesting results have already emerged.
From a theoretical perspective, the predicted electron bands really should be there; this claim holds up to scrutiny.
Experimentally, however, creating these samples seems to yield no superconductivity at all, with multiple studies failing to replicate what Lee and Kim asserted that they saw.
Some of the problems with the Lee and Kim studies have also come to light:
- they only apply very small currents and voltages to their samples,
- they have practically no data points that examine the transition to their alleged zero-resistivity state,
- and the “magnetic levitation” that they observe is much more consistent with boring old diamagnetic repulsion (as in the levitating, non-superconducting frog shown in the video below), rather than the traditional flux-pinning levitation associated with superconductors and the Meissner effect.
We have a saying that extraordinary claims require extraordinary evidence, and it’s looking more and more like the work that Lee and Kim have conducted has been solid from a theoretical point of view, but sloppy from the only point of view that truly matters: the experimental one. Based on what we’ve seen so far, this doesn’t appear to be a case of fraud as with Ranga Dias, but rather a case of wishful thinking and insufficient data getting the better of us. Other scientists have chimed in with their thoughts about this research as well, and they’re similarly skeptical: the evidence just isn’t there for this material to be superconducting at all, much less at room temperature. Instead, it’s more akin to cold fusion research: where if you make some untrue assumptions, the phenomenon should occur, but the “interesting” predictions that you’re making don’t actually correspond to what you’re hoping nature will bear out.
With further replication attempts underway, we’ll soon find out whether the LK-99 material:
- superconducts at all,
- superconducts at room temperature,
- whether the observed “levitation” is due to diamagnetism, the Meissner effect, some other magnetic phenomenon, or is an experimental error,
- and whether the (controversial) theories of superconductivity used to predict the phenomenon are valid, or whether they’re simply incorrect predictions that are contradicted by experiment.
In science, you can theorize and calculate all you like, but in the end, it’s the experiments that determine what is real and what is not. A single positive, robust replication will be all that it takes to usher in a new era of materials science and could literally change the world, but until that confirmation arrives, this is an extraordinary claim that may have captured our collective imaginations, but hasn’t yet met the needed standards for “extraordinary evidence” for any responsible scientist to accept it.
This article was reprinted with permission of Big Think, where it was originally published.






