This article is an installment of Future Explored, a weekly guide to world-changing technology. You can get stories like this one straight to your inbox every Saturday morning by subscribing above.
It’s 2025, and you just paid $400 to have your entire genome sequenced — something that would have cost $10 million just a couple of decades ago. Armed with every base in your biological blueprint, you can now work with your doctor on a plan to counter your genetic predisposition to certain diseases, increasing your chance of living a longer, healthier life.
Personal genomics
Human DNA is made up of pairs of chemical bases — either adenine (A) and thymine (T), or cytosine (C) and guanine (G) — strung together like the rungs in a long, twisted ladder. Genes are short segments of DNA, and your genome is all the genes in your body.
Genes provide instructions for your cells, usually telling them how to make certain proteins, but an abnormality in the order of the bases in a single gene — a TA pair where there should be a CG, for example — can be enough to cause a disease.
Human genome sequencing is the process of writing out all ~3 billion bases in a person’s genome in order. It has played an integral role in helping scientists understand genetic diseases, and after decades of development, it’s finally getting cheap enough for the mass market.
To find out what that means for the future of healthcare, let’s take a look back at the history of genome sequencing and the startup that wants to use it to improve your health — and maybe even help you live longer.
Where we’ve been
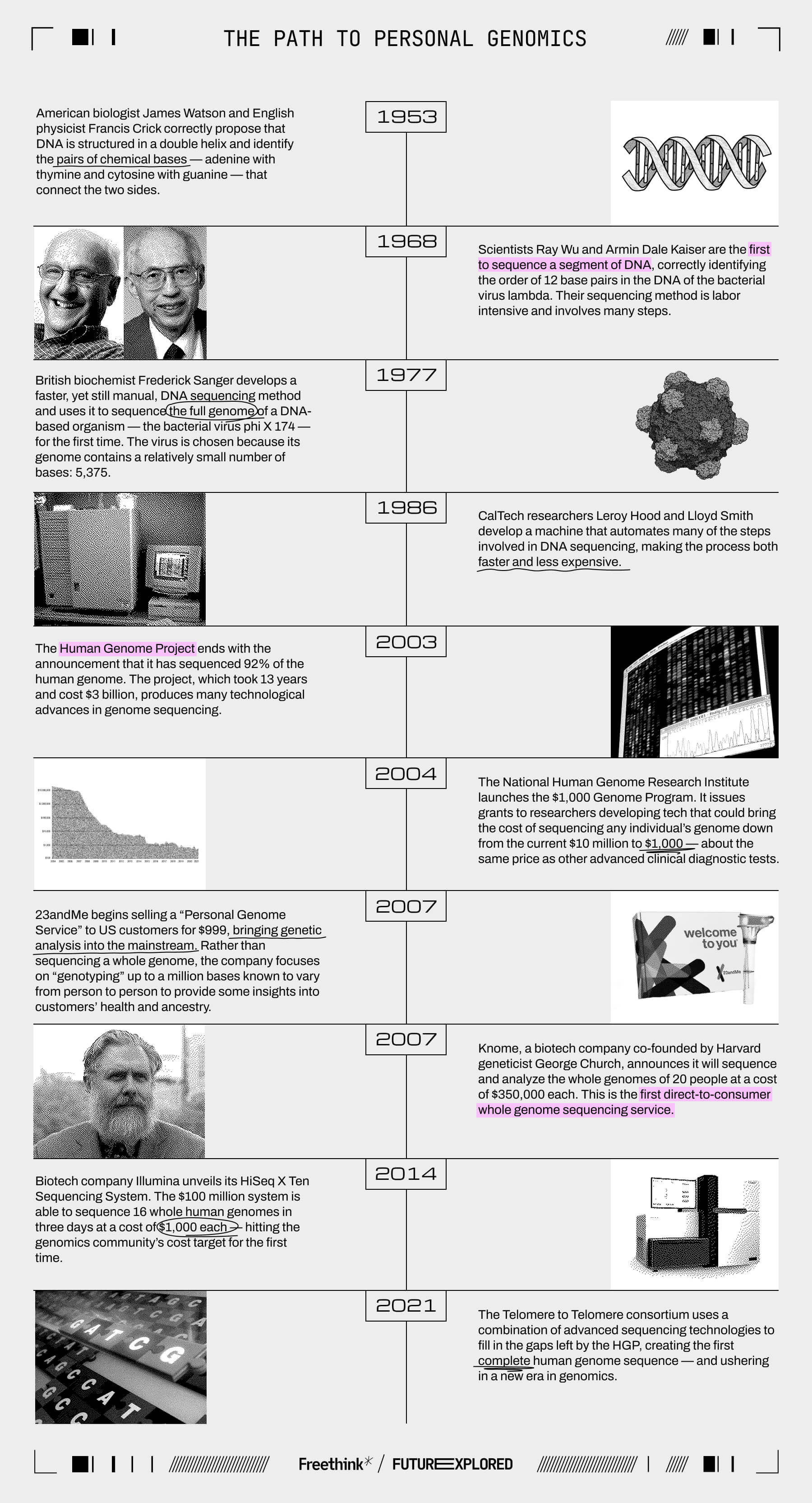
Where we’re going (maybe)
In the decades since the Human Genome Project ended, the cost of sequencing a whole genome has fallen exponentially — just last year, Illumina introduced a new machine it says can get the job done for just $200.
At the same time, the amount of genome data available for analysis has increased significantly. In 2023, the UK Biobank announced that it was making a database containing the genome sequences of 500,000 anonymous volunteers, along with information from their health records, available to approved researchers.
That means we not only have the ability to sequence a person’s genome at a relatively low cost, but also enough examples of genomes and life outcomes to put what we discover into some sort of context.
Add in recent breakthroughs in gene therapy, which could be used to correct “bugs” in our genetic code, and you get a society on the brink of a new era in medicine.
Nucleus Genomics hopes to be a leader of this era.
In August 2024, the Brooklyn-based startup launched its first product: a whole genome sequencing and analysis service that costs just $399. A $39 annual membership fee gets the customer access to new features and reports as they become available.
“Nucleus is the all-in-one genetic test,” Kian Sadeghi, the company’s founder, said at the time of launch. “The promise of the Human Genome Project has finally arrived.”

Sadeghi learned firsthand about the power of genetics when he was just five years old and his teenage cousin unexpectedly died in her sleep. Doctors would later suspect that she’d had long QT syndrome, a rare cardiac disorder that’s usually genetic.
It wasn’t until Sadeghi was an undergrad studying computational biology at Penn, though, that he got the idea for how he might be able to harness genomics to help others avoid such tragedies.
“I was in BIOL 221, and the professor brought up a chart showing the decreasing cost of whole genome sequencing,” Sadeghi told Freethink. “It started at $1 billion, and at the time — 2020 — it cost $1,000.”
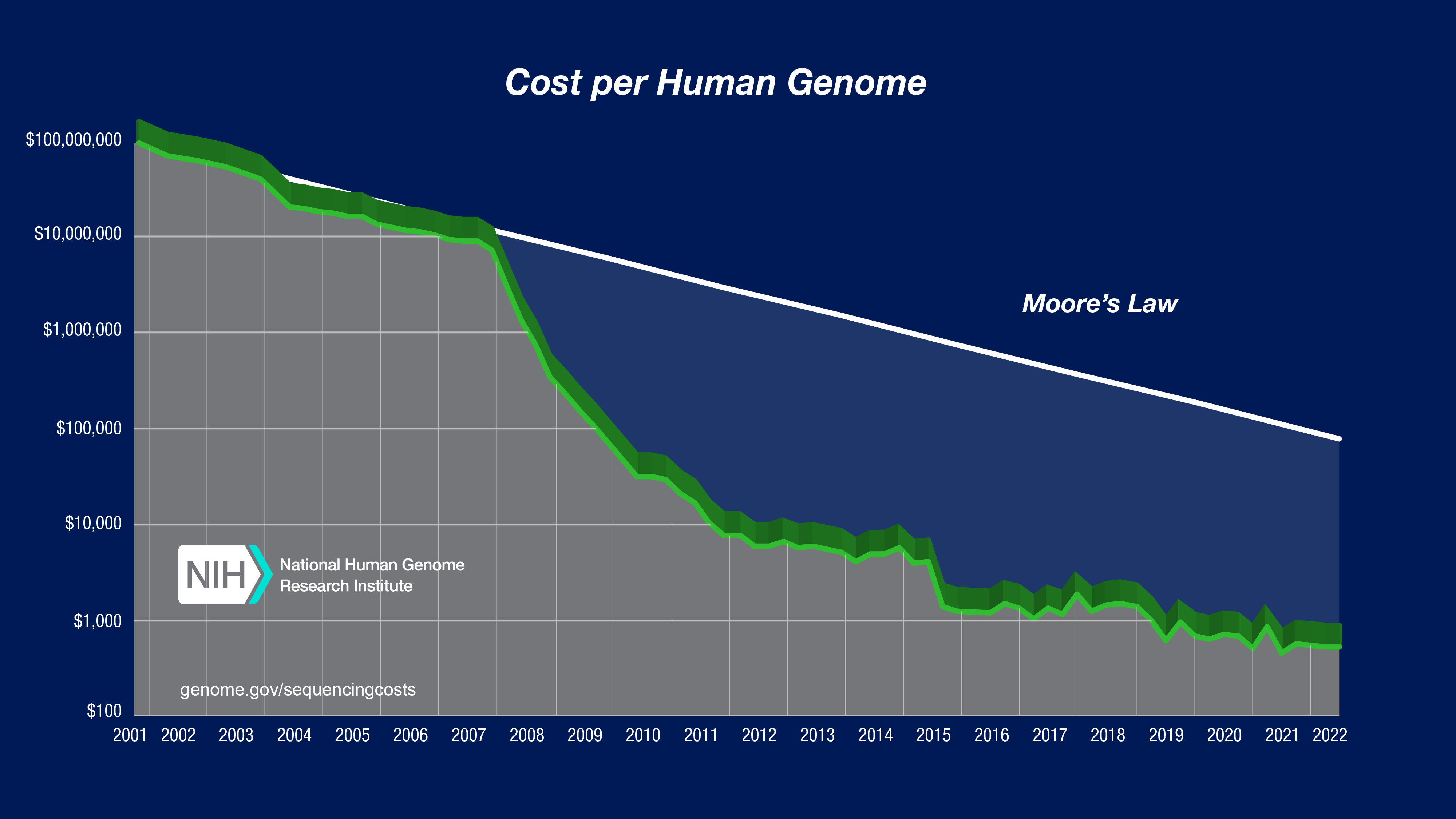
“I thought to myself [that] it’s an inevitability that this price is gonna converge on zero and every single person in this country is going to be able to benefit and gain from the entirety of their DNA — not just a small fraction of it, but all of their DNA,” he continued. “With that in mind, I dropped out of school, moved back into my bedroom in Brooklyn, and I started building.”
By 2021, Sadeghi was ready to bring Nucleus Genomics out of stealth, and by 2022, the startup had raised more than $18 million, with Reddit co-founder Alexis Ohanian’s venture capital firm Seven Seven Six leading the funding.
“Can’t wait for this platform to deliver next-level analysis that improves — and can even save — lives,” Ohanian later tweeted.
Customers use Nucleus’ testing kit to collect samples of their DNA through simple cheek swabs. Nucleus then sends each sample to Illumina for sequencing. The entire process happens in the US on US machines, and customers are automatically opted out of having their data shared with any third parties (though they can choose to participate in research programs).
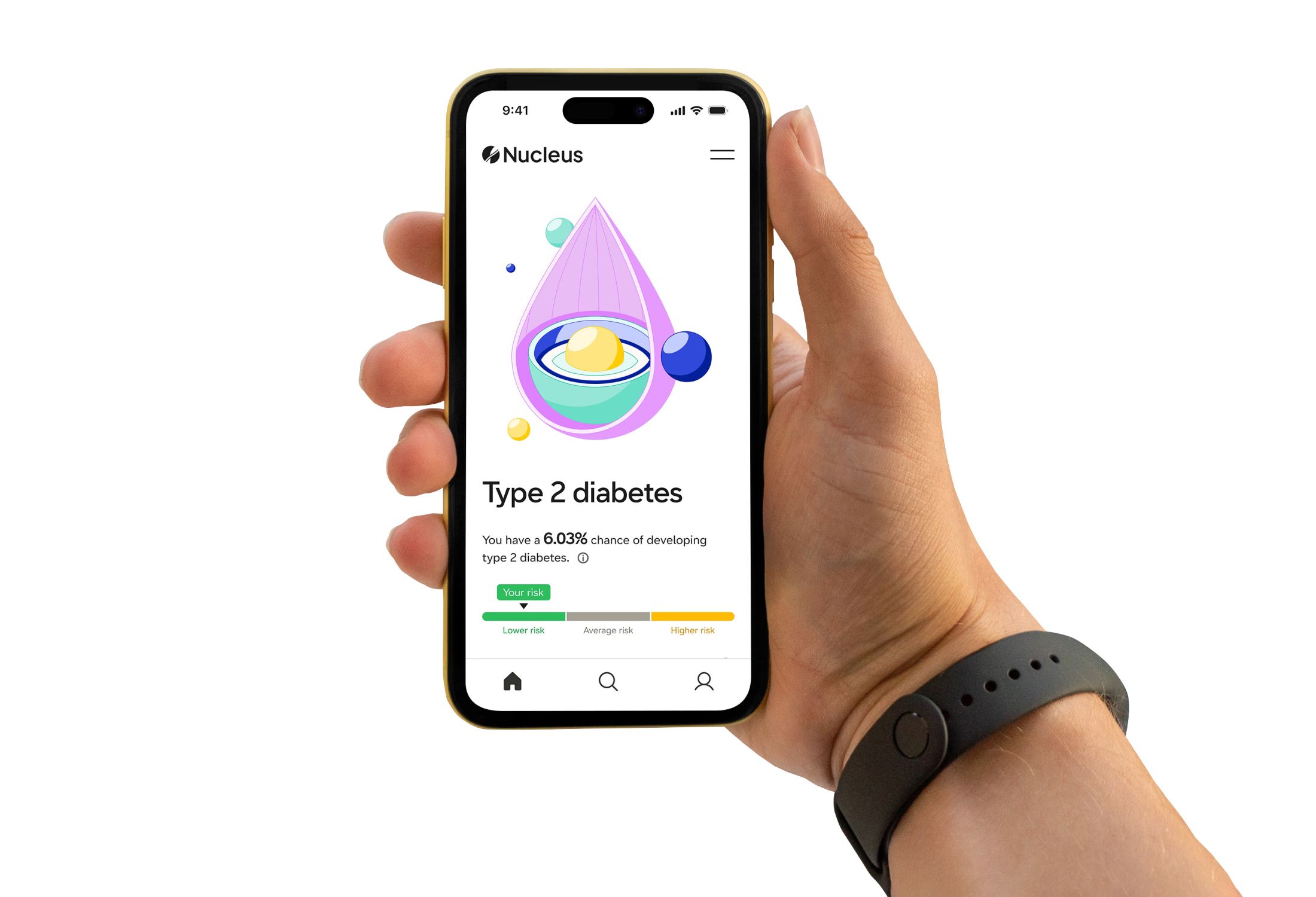
About six weeks after submitting their samples, users are notified that their report is available online. It will let them know about any variants in their genome — bases that differ from the standard human genome — and information on how those variants could impact their health.
The report also includes common genetic scores (aka, polygenic risk scores) for more than 20 diseases. Those predict their risk of developing the disease relative to other people of their same ancestry. They are generated by Nucleus’ algorithms, and they take into account multiple genetic variants and the latest peer-reviewed research.
Nucleus’ reports also provide users with scores indicating their absolute disease risk. Those consider genetic as well as non-genetic information, such as a person’s environment, age, and BMI, when predicting their likelihood of developing a certain disease compared to others who share their ancestry.
“It is no longer those who are the most strong that are going to survive and live the longest. It is those who are the most curious”
Kian Sadeghi
Nucleus recently added Nucleus Traits and Nucleus Longevity to its reports, too. Traits predicts a person’s genetic predisposition for certain characteristics, such as baldness or a willingness to take risks, while Longevity predicts their likelihood of living past the age of 80 compared to others like them.
Sadeghi announced the latter feature at the “Don’t Die Summit” (put on by Blueprint CEO Bryan Johnson), with a prediction that whole genome sequencing could lead to a new model of human evolution: Survival of the Curious.
“It is no longer those who are the most strong or have some sort of endurance or physical prowess that are going to survive and live the longest,” he told the audience. “It is those who are the most curious, who go back to the foundational layer of their DNA and unlock the insights so they can live a long and healthy life.”
Just being curious enough to have your genome sequenced won’t lead to a longer or healthier life, though — for that to happen, people will need to be able to do something with the insights gleaned from Nucleus’ reports.
That’s where the idea of “life optimizations” comes into play. These are actions a person can take to try to combat their predisposition to certain diseases, and Sadeghi places them in three categories.
“One is what I call ‘urgent and actionable,’ which is like what my cousin was deemed to have died of — if you know you have long QT, you can take a beta blocker that will reduce your risk [of a cardiac event], so it’s both urgent and also extremely actionable,” he told Freethink.
“There’s really no such thing as a genetics company — there are only healthcare companies.”
Kian Sadeghi
Others are less urgent, but still actionable. For example, if you found out you carried a faulty copy of the BRCA1 gene, which puts you at high risk of developing breast cancer, you could be more aggressive with your screenings or even undergo a double mastectomy to reduce your risk.
Depending on the condition, you might even be able to receive gene therapy to fix a faulty gene, though such treatments are currently rare.
The third category includes conditions like Alzheimer’s, and while there’s nothing a person can do right now to dramatically reduce their risk of developing these diseases, Sadeghi believes that knowing that one is genetically predisposed to them can still be worthwhile — it might inspire them to keep up with the latest research or to try to live a generally healthier lifestyle.
Nucleus has partnered with telehealth company SteadyMD to make appointments with genetic counselors, who can help people identify appropriate life optimizations, available to its customers for an additional fee, but the startup aims to eventually play a larger role in helping people translate the results of their reports into longer, healthier lives.
“I like to say that there’s really no such thing as a genetics company — there are only healthcare companies,” Sadeghi told Freethink. “The vision is for us to be able to thoughtfully, in a regulatory compliant manner, provide people with appropriate next steps depending on their genetic result.”
Whether those next steps would be on shaky or solid ground is hard to say, though.
While Illumina’s tech ensures that Nucleus’ genome sequences are 99.9% accurate, the company’s analysis doesn’t fall under the FDA’s jurisdiction, so it doesn’t have to disclose how it arrives at its predictions or prove their accuracy. It’s possible that your genes make you more likely to develop heart disease than 75% of people with your ancestry, but there’s no way to know what your future holds until you do (or don’t) get sick.
The utility of polygenic risk scores, in general, is still unclear — research suggests their ability to accurately predict disease risk is limited, particularly for people who aren’t white since today’s genome databases lack diversity. Because Nucleus’ absolute disease risk scores include factors beyond genetics, they could be more accurate, but again, it’s hard to say for certain.
Accurate or not, some worry that direct-to-consumer genome sequencing and analysis services could actually do more harm than good — people might spend money on screenings they don’t actually need, or experience anxiety because they think they’re at high risk of developing a disease they can’t really do anything to prevent, like Alzheimer’s.
“It’s an inevitability that every single person has their entire human genome on their iPhone.”
Kian Sadeghi
Nucleus isn’t alone in facing these issues — the entire genomics community is still wrestling with how to translate increased access to the human genome into something that will actually help people live longer, healthier lives.
Growing the size and diversity of our genome databases — something that’s well underway — should help in this mission, as should the development of more powerful AI tools that make it easier for researchers to make sense of these giant troves of data.
The bottom line, though, is that report after report predicts significant growth for the consumer genomics industry over the next decade, and if Sadeghi gets his way, Nucleus will be the company meeting the bulk of the demand for these services.
“It’s an inevitability that every single person has their entire human genome on their iPhone,” Sadeghi told Ohanian in 2022. “You have to wonder, with this ubiquitization of genomic data, who’s going to analyze it? Who’s going to store it? Who’s going to maintain it?”
“It’s going to be Nucleus,” he continued. “The name of that app is going to be Nucleus.”
We’d love to hear from you! If you have a comment about this article or if you have a tip for a future Freethink story, please email us at [email protected].